Ahh the joy of mixing metals in a closed water loop…:) While many water coolers have had excellent success with running copper/brass/nickel over the years with plain water, we have seen many examples of where certain conditions result in not so favorable results. While we often call copper/brass loops a “Similar” metals loop, I think we are also forgetting that “Similar” is not the “Same” and we have a LOT more than just copper and brass in our loops. Your typical water cooling loop has a mixture of copper, brass, nickel, and tin. Note, that I think I’m the first example of the “TIN” corrosion by an unintentional experiment I had been carrying out over the last year or so..:)
The manufacturers pretty much all say “use our coolant” which includes corrosion inhibitors, yet we persist in thinking nothing is wrong with this mixing of “Similar” metals. I’m not a corrosion expert by any means and have typically had the same or similar good success without the use of inhibitors. I am however becoming more of a believer of corrosion potential as these repeated problems persist and as I have now experienced a recent loss myself.
Last year when doing my fan testing series, I filled up two radiators with water as part of my testing rig templates. One was a Swiftech MCR120, and one was a Hardware Labs SR1 140. Upon digging those radiators out in preparation for my radiator testing bench rebuild, I noticed that the MCR was still full of water, but the SR1 140 was empty. I also noticed what appeared to be water stains on the bottom of the SR1.
Could this be corrosion? I thought..




Oh my, my SR1 has become a victim of corrosion!!
But I thought the idea was if you run copper/brass loops, corrosion wasn’t possible?
Well…it is..
My SR1 is now a leaking sieve, so I decided to do a little digging in on galvanic corrosion. I think most people including myself have been thinking about corrosion between copper/brass/nickel, but I don’t think we have been thinking about the solder in radiators.
What is Galvanic Corrosion?
Per Wiki:
Galvanic corrosion is an electrochemical process in which one metalcorrodes preferentially to another when both metals are in electrical contact and immersed in an electrolyte. The same galvanic reaction is exploited in primary batteries to generate a voltage.
So it’s not all bad..after all Galvanic corrosion is what starts your car in the morning..:)
What is needed for Galvanic Corrosion?
Per the corrosiondoctors.org:
Electrochemically dissimilar metals must be present
These metals must be in electrical contact, and
The metals must be exposed to an electrolyte
Of particular interest to me is #2, I didn’t realize that the metals had to be in electrical contact, but that does explain a few things I’ve been seeing.
Now to make sense of my SR1 loss:
Ok, so in a closed loop of water, while water is initially non-conductive, it only takes a short time in a water loop to become contaminated and conductive. Once conductive it now satisfies the “Electrolyte” criteria. #3 is done. The stagnant condition (and not at all typical) probably made this many times amplified.
Also the metals must be in electrical contact. In my radiator example the soldered connection of the radiator fins is clearly a good metal contact. #2 is satisfied.
And finally they must be dissimilar metals:

According to the corrosiondoctors.org anodic index, it appears a normal copper solder radiator has about a .30 galvanic potential.
Yep, seems to make sense I guess. How about a few other examples others have shared:
ALUMINUM/COPPPER
Aluminum and Copper in direct contact. Pn0yb0i gave an example of what running an aluminum/copper block can do after an extremely long 4 year run here. He was going to reuse the block after cleaning, so I ask him if I could use some of his pictures if I sent him a replacement block sample for free. He accepted happily and I feel better that he’s got a new block too..:) Anyhow, here are a couple of photos that he shared and gave me permission to use.
He said he used distilled water plus pentosin and purged every 2 months.
And to compare the anodic index between copper and aluminum.
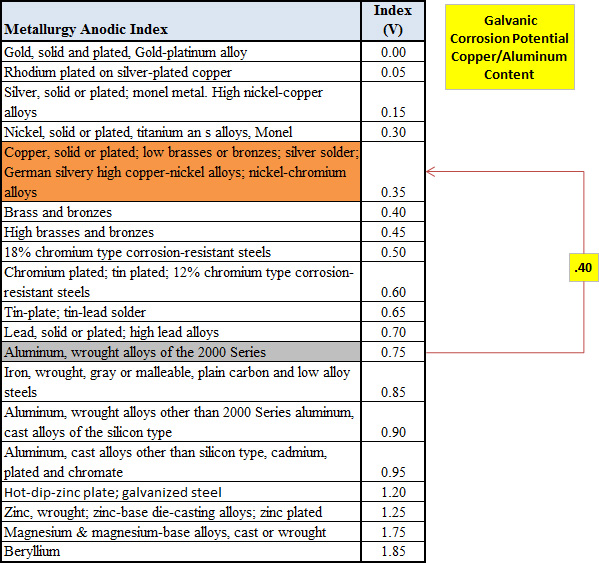
In general most manufactures have given up on attempting to make aluminum/copper blocks, which has led to eliminating that problem.
However, as water cooling has become “Art” as much as it is performance, there has been a dramatic increase in Nickel plating of blocks. It is handy not having to deal with tarnished copper and a lot of people like the shiny surface of Nickel plating which in itself has caused problems as well…
Nickel Plating
The hot topic in the forums has been in regard to nickel plating failures. Manufacturing processes have been improving regarding the plating quality. Electroless plating is the latest preferred method which is supposed to plate the parts more evenly.
If you look at the anodic index again and compare nickel to copper, you can see it is actually very similar in index meaning their corrosion potential is very small. In the case of metal corrosion, opposites attract and nickel/copper are very similar.
But….the difference is still there.

We have seen failures on blocks and we have seen failures on fittings. The one commonality I have seen in all the various forum examples is “stagnant” water. Just like my radiator example, where you normally see plating fail, is where water sits still. I believe this stagnant condition is what promotes the #3 electrolyte condition. The longer the water sits still near metals the more contaminated and electrolyte like it gets. We typically see plating failures between surfaces such as the GPU block and acrylic or delrin top. We also see it between CPU nozzle plates and the CPU block bases plated in nickel. In fittings we see the plating failures at the threads…again where the water is stagnant.
The other reason I think the small index still causes problems is simply due to the plating being very thin it just doesn’t take much to show. Also since copper is the anode to nickel, it works in an undermining process where the copper goes away, and the nickel flakes. Also as the copper goes away and undermines the nickel, it creates a pocket where that electrolyte enhancement (stagnant water) grows even faster.
I do think a “Perfect Plating Job” could avoid the issue with plastic tops, if there was a perfect nickel plating over the copper block and that was the only metal in direct contact, you will have essentially removed the “electrolyte” variable. If there is no way for the electrolyte (water) to get between the copper and nickel, then life is peachy. I just don’t think plating is ever perfect. Any little microscopic pin hole, scratch, or thread wearing into the plate will expose the copper allowing the reaction to occur.
What is the problem?
- We are mixing metals.
- Some of the mixed metals have direct electrical contact.
- Our water is becoming an electrolyte with stagnant water conditions in some areas.
Sacrificial Anodes
One thing that hasn’t really been explored much in water cooling is the use of sacrificial anodes. These are used quite regularly for corrosion applications where the idea is to make the electrolytes go after a more active metal instead of the metals you are trying to protect. The anode need to be in electrical contact with the other metals and will over time corrode and need replacement. You see them in household water heaters and on ships in saltwater, and bridges along the coast. Most industries that have some sort of corrosion problem lean toward either or a corrosion inhibitor or some sort of anode to provide that protection.
I don’t see why you couldn’t have some sort of zinc barb insert or something that could be easily replaced though. I’m not quite sure what sort of deposits the zinc would make, but it should theoretically work in preventing corrosion from occurring. I’m not sure???
I could see that as being a possible solution for folks that would rather not run anything than water. That’s what we do for water heaters (inhibitors not possible), why not for water cooling?
Anyhow, not sure if sacrificial anodes would work or not, but I’m really curious to try. It could be a solution for giving plain water loops corrosion protection without the fuss of a coolant with inhibitors. You would just need to attach a piece of zinc to each of the mixed metals blocks/rads and see what happens.
Conclusion
I don’t think it is possible to completely stop galvanic corrosion from occurring, but we can reduce it by:
- Eliminating direct electrical contact of dissimilar metals (Plastic top/unplated copper base blocks)
- Reduce Electrolytic Conditions – Reduce areas where water is stagnant, flow is your friend. Regular maintenance and complete cleaning of the block/pieces probably helps too.
- Improve plating processes and increase plating thicknesses.
- Slow the process with corrosion inhibitors in the fluid
- Slow the process using a sacrificial anode in system running plain water.
Cheers!
Martin




Be interesting to see the results of a sacrificial anode in a closed loop as your water heater and Evenrude are both open loops with a hypothetically endless supply of fresh/new water so the water is not building up a concentration of zinc, (or w/e the sacrificial metal used is) and possible redepositing it elsewhere.
It could leave a mess I suppose, but we likely are getting that same exact thing with the dissimilar metals we have. Particularly the Tin/Lead>Copper in folded fin radiator designs is a pretty high index, it’s just not something we get to see happening an I suspect it’s also not as quick because of the turbulent free flowing conditions in those end tanks. I just figure if anodes are acceptable in our water heaters that well all shower and bathe in, maybe it would work fine for a water cooling loop too. Just have to try and see I guess, but I’m not sure where the best place is for the anode if it has to be attached electrically. I could see it being some sort of barb insert on radiators which has metal contact throughout, but what about all those blocks that have plastic tops. There the top isolates direct metal contact from the barbs, so you have to place it somewhere on the block. I suppose it could just be a small zinc washer of sorts with a small screw that would attach to the block through the barb hole or something easily accessible.?
Seems to me like we need an overhaul in the water world. Redesigns in blocks, better solder used in radiators, or a complete dropping of DI water and solely using anticorrosive coolants. We can keep corrosion at bay, but we’re honestly creating situations in our loops that foster corrosion.
I guess I have a new appreciation for the reality of corrosion after loosing the radiator, but I definitely don’t think corrosion is completely avoidable with current designs. I suppose you could make a copper only system of barbs and blocks and solderless radiators, but that would be pretty expensive.
I think it’s more of a need to better understand the variables that increase risk. We know a bare copper block with acetal or acrylic top is pretty safe. There is no electrical contact with another metal there. Also we do have corrosion inhibitors readily available, but I’m still a water only preference myself even though I know there are risks.
I think we have been in denial about copper/brass/nickel/tin going on the assumption that they are like enough metals that there is zero risk. They are similar, but not the same, so there is potential there with the right condition.
“We know a bare copper block with acetal or acrylic top is pretty safe. There is no electrical contact with another metal there.”
There is no METALLIC contact, but once you fill the loop with coolant you’ll have ELECTRICAL contact between all metal parts in that loop.
a) You use the Anodic Index for metals in metallic contact.
b) You use the Standard Electrode Potential for metals immersed in an electrolyte.
Once people understand that they will stop using killcoils and nuke.
c) That “stagnant water” thing is called crevice corrosion. Very common.
As for the HW Labs radiator it could just be bad workmanship. I once punctured a radiator with a too long screw and it looked exactly like that, it had nothing to do with corrosion at all.
Sacrificial anodes has no place in a closed PC watercooling loop. Use a coolant with corrosion inhibitors and anti-biofouling properties.
The car industry mix metals like copper radiators, aluminum block, iron etc. The user manual INSTRUCT the owner to use a coolant with antifreeze and corrosion inhibitors. I don’t why so many self appointed experts advise people to use distilled water with conducting additives like nuke when the world largest industry instruct their customer to do the opposite.
Thanks for the info, checking out the standard electrode potential thing. Cheers!
Martin
If you have a CPU block with copper bottom and aluminum housing, and they are in metallic contact, you should use the Anodic Index. Swiftech actually made such water blocks some 5-10 years ago.
If you have a copper block and an aluminum block in the same loop it will work fine as long as there is no electrical connection between them. But often it will be.
Think battery. If the plus and minus is electrically connected (short circuited) with a wire it will not flow any current unless there is battery water in the battery cells. If you have battery water in the the battery cells you must be careful not short circuit the battery poles.
Back to PC watercooling: Use nickel/copper blocks and a copper radiator. There’s a good chance they will be “grounded” to the PC case, the radiator certainly will. Use a aluminum reservoir, fasten it to the PC case and put a silver coil in it. Now we have a several different metals electrically connected through the chassis. All we need now is to fill the loop with distilled water, add some conducting stuff like Nuke and we have a nice electrochemical cell. Check the Standard Electrode Potential where silver is and you’ll understand how potentially harmful it can be.
A sacrificial anode ….. i think you might be onto something good here Martin. I’m going to see if anyone else has thought of that before. But simply put, all metals rust (or oxidise) sooner or later. You can’t stop a metal from rusting, short of completely isolating it from air or water..
Take a steel nail and leave it on a shelf: it will rust eventually.
Chuck that nail in water and it will rust even faster due to the water being an oxidising agent.
But if you chuck a piece of zinc in the water along with the nail, that will considerably slow down the steel nail from rusting and the zinc will rust (or oxidise) faster then normal.
The nail has become a cathode and the zinc has become an anode.
I am definitely dropping a chunk of zinc into my next loop, GREAT IDEA Martin!!
I think no matter what you do…if you have a DIY water-based cooling solution in place today it’s a necessity to do the “Jiffy-Lube” type service every few months. Most of us do that anyway…replacing tubing, adding blocks, changing out video cards and then cases…In the two-years I’ve been in W/C I think I’ve never run more than a few months w/o major change. That said…automobile manufacturers seem to have got their issues worked out…typically a car’s radiator and cooling system never needs to be replaced so I suspect their metallurgy is up there (all aluminum?)…since they control the manufacturing of the entire car maybe what we need in this industry are some sort of report-able standards so you know if you mix Radiator A with CPU Block B…it’s a good match. Some kind of hard-core analysis that states you’ll get a two-year life expectancy if you mix these two devices…and a week-and-a-half if you mix these.
[…] […]
A good read Martin! Also the block you sent is bangin!
Thanks! Glad you got a new block in there..:)
There is little to no evidence that the small amount of EG or PG found in most premix coolant will slow down the process of corrosion. In fact, there is little to no evidence that large amounts effect the rate of corrosion in PC water cooling.
Proper maintenance and inspection every so often is good preventive tool. Letting your loop run for years is not te worlds best idea.
“Plastic top/unplated copper base blocks”
Hope you meant aluminum top, or I just got incredibly worried.
I said it correct. If you have a plastic top like acetal or delrin on a solid copper base, those have been pretty solid. There is no dissimilar metals in direct metal to metal contact on those. You do get some tarnishing problems, but nothing a little ketchup or lemon water won’t fix up shortly. Plated blocks however I think to have a bit more risk. I don’t think it’s that the rate of corrosion is that much different, it’s just that it only takes a tiny bit of corrosion to flake some plating and become very obvious. A little corrosion on a solid piece of metal just isn’t very visible and perceived as nothing going on. Also the remedy to a little corrosion on solid copper is pretty easy where a failure on plating isn’t something you can fix with any home brew method other than sand blasting the rest of the plating off.
I’m still not sure what to think about the fluids electrical conductivity serving as that “Direct electrical contact” point the referenced articles mention. We do know that it’s conductive, but why is the corrosion mostly showing up at areas of stagnation if there wasn’t something affecting the water’s electrolyte capability.
I ordered a couple of water test meters to explore this further..:)
Also, if stagnant water is an issue, somebody should work on a pump that stays on at a low flow rate when the pc is off. Or an atx connector mod or something, that just sends power to the pump when the pc is off.
Another thing that is very important is proximity. In my Koolance RP-452×2, I have Koolance’s nickle plated bleed pipe installed. I used to keep my silver kill coil in the reservoir, which was a mistake. The nickle plating on the bleed pipe would corrode to a nasty red in about a week, with the first signs showing up within 24 hours. My kill coil is now in a section of my tubing, and the nickle bleed pipe is corroding at a much slower pace. After about a month the signs of corrosion on a new pipe are just starting to show up.
This also points out that we might need to consider that our silver kill coils, while effectively killing living organisms, might also be the source of galvanic corrosion.